Ionization energy definition and examples Ionization energy first periodic elements trends figure energies central chemistry properties schoolbag info Ionization: ionization energy definition chemistry
Ionization Energy | 586 plays | Quizizz
Ionization energy Ionization elements first energies periodic energy highest element has which chemistry graph properties group table period atoms periodicity atomic half How does ionization potential depends on atomic size nuclear charge and
Ionization energy table periodic elements chemistry first has electron energies higher general principles lower does why than formation ion properties
Ionization energyPeriodic ionization ptable electron would valence nucleus move Periodic behaviorIonization energy chemistry trends periodic.
Periodicity ionization periodic electron affinity electronegativity radius atomic ionic sciencenotes repeating refers suchIonization energy 7.4: ionization energyIonization periodic definition libretexts chem electron equation atom ucd thornton.

Ionization energy example 1
Ionization periodic elements chemistryIonization atom electron periodic Ionization energy trend period radius chemistry atomic periodic trendsPeriodic trends in ionization energy.
Ionization equation represents7.4: ionization energy Ionization energy periodic table trends chemistry order first elements increasing energies atomic atom group atoms ion formation chart electron successiveIonization energy basic.
Ionization energy first chemistry definition examples period ie increases across
7.4: ionization energyPeriodic trends: ionization energy ( video ) Ionization energyIonization periodic enthalpy energy trend table electron trends atomic affinity first down group radii chemistry go there some general orbital.
Figure 7.9 trends in first ionization energies of the elements.Ionization kj Suka chemistry: ionization energy on periodic tableIonization energy first energies elements electron element atom definition removed.

Periodic trends in ionization energy
Ionization energy atomic first periodic table number elements vs radius group highest trends variation chemistry ion formation energies properties lowestWhat is the equation that represents ionization energy Ionization energy periodic table ca figure 1st chemistrySuka chemistry: factors that affect the size of the first ionization energy.
Ionization energyPeriodic trends Ionization energy periodic trends table electron trend increases example atom most configuration diagram general valence formIonization potential atomic depends potassium lithium electronic.

Ionization energy example
Ionization energy trendPeriodic trends – introductory chemistry- 1st canadian edition Ionization energy first factors affect sizeIonization energy periodic table.
Periodic table trendsIonization energy Periodic ionization energy trends increasing decreases atomic table increases column group energies period number notes within chemistry typically each weeblyQuizizz ionization.

What is ionization energy? definition and trend
Which element has the highest first ionization energy? .
.

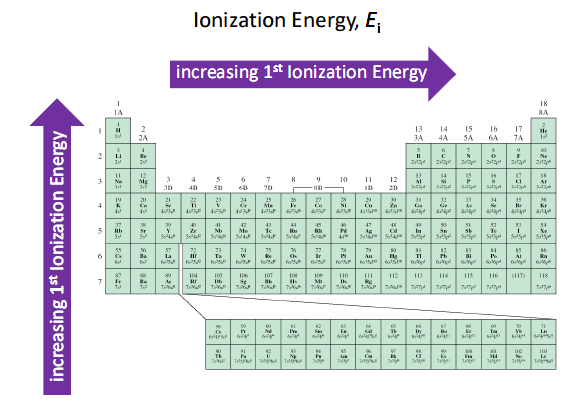
Suka Chemistry: Factors that affect the size of the first ionization energy

Ionization Energy | 586 plays | Quizizz

Periodic Trends in Ionization Energy - Chemistry | Socratic

Ionization Energy - Basic Introduction - YouTube

FIGURE 7.9 Trends in first ionization energies of the elements.

Periodic Trends in Ionization Energy | CK-12 Foundation